Abstract
Purpose
A nonmyeloablative (NMA) regimen of fludarabine and 200cGy TBI combined with post-grafting immunosuppression with mycophenolate mofetil (MMF) and a calcineurin inhibitor allows for allogeneic hematopoietic cell transplantation (HCT) from HLA-matched related or unrelated donors in older patients and those with comorbidities affected by myelodysplastic syndrome (MDS) or myeloproliferative neoplasms (MPN). Results of phase I/II studies in patients with chronic myelomonocytic leukemia (CMML) or MDS/MPN have been disappointing, however, due to high incidences of relapse or graft failure (together termed HCT-failure). We hypothesized that escalating the TBI dose may decrease relapse and ensure engraftment. We performed a phase I/II TBI dose-escalation trial and compared the rates of HCT-failure.
Methods
This was a study conducted at three transplant centers. Patients ages 50-75 or <50 (median age 66) years with high risk comorbidities (Table 1) received NMA conditioning followed by HCT, with TBI dose escalation:
Arm A - patients with MPN or low-risk MDS (RA-RARS-RCMD) or PNH
Arm B - patients with high-risk MDS (RAEB-1) or CMML
Patients with MDS/MPN could not have received myelosuppressive chemotherapy; patients with CMML who had progressed could have received myelosuppressive chemotherapy before HCT to reduce marrow blasts to less than 5%.
Patients were enrolled in groups of 6; dose escalation rules were imposed for HCT failure >20% before day 200 on Arms A and B. Stopping rules were imposed for nonrelapse mortality (NRM) at day 200 of >25% in Arm A and >35% in Arm B.
The TBI dose levels were:
Level 1: 300cGy
Level 2: 400cGy
Level 3: 450cGy
All patients received fludarabine 30/m2/day IV x3 days on days -4 to -2. TBI was administered on day 0 followed by infusion of G-CSF mobilized PBSC from HLA-matched related (n=30) or unrelated (n=47) donors. Post-grafting immunosuppression with MMF and cyclosporine was administered.
The primary endpoint was a decrease in the incidence of day200 HCT-failure to <20%; secondary endpoints included overall survival (OS), progression-free survival (PFS), relapse incidence (RI) and NRM.
Results
The study enrolled 77 patients with 36 patients in Arm A and 41 patients in Arm B. Median follow-up is 56.3 months among surviving patients.
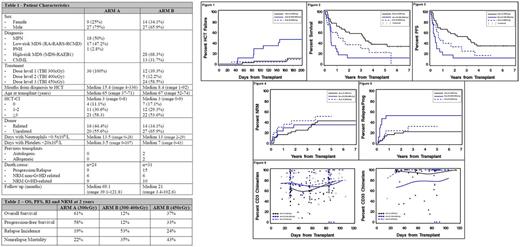
The primary endpoint (Figure 1) was reached on Arm A at dose level 1 (300cGy TBI) with a cumulative incidence of day200 HCT-failure of 11%.
The primary endpoint was not reached in Arm B at dose levels 1 and 2, with dose escalation being triggered at 12 and 5 patients respectively. The endpoint was reached on dose level 3 (450cGy) with a cumulative incidence of day200 HCT-failure of 9%.
See Table 2 and Figures 2-5 for OS, PFS, RI and NRM.
Cumulative incidence of grades III-IV acute graft-versus-host (aGvHD) by day100 was 17% in Arm A, 12% in Arm B for dose levels 1-2, and 9% in Arm B for dose level 3. Chronic graft-versus-host (cGvHD) cumulative incidence at 1year was 44% in Arm A, 35% in Arm B for dose levels 1-2 and 29% in Arm B for dose level 3.
Regarding chimerism analysis, no statistically significant differences were seen among the different arms (Figure 6).
Summary and Conclusions
In Group A (MPN / low-risk MDS), increasing the TBI dose from 200cGy to 300cGy reduced the day200 HCT-failure rate from 31% in previous trials to 11%. As a result, the OS and PFS was 61% and 58%, respectively, at 2 years. Similarly, for Group B (high-risk MDS / CMML), the day200 HCT-failure rate was reduced from 58% in previous trials to 9% when 450cGy was used. This resulted in an OS and PFS of 37% and 33%, respectively, at 2 years. TBI doses of 300cGy and 400cGy were insufficient to reduce HCT-failure in this high risk group.
In conclusion, increasing the TBI dose can lead to a higher success rate in this setting of nonmyeloablative conditioning by reducing both relapse and rejection. Further studies are necessary to decrease nonrelapse mortality, especially among patients affected by high-risk disease. Current trials using targeted radioimmunotherapy are currently being investigated towards this end.
Flowers: Pharmacyclics: Consultancy. Maloney: Kite Pharmaceuticals: Other: Advisory board; Celgene: Other: Advisory board; Juno Theraapeutics: Other: Advisory board, Patents & Royalties, Research Funding; Roche/Genetech: Other: Advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal